Individuals with hearing loss use both personal hearing devices and assistive listening systems to maximize their communication access.
Although underused, the most common technology utilized by people with hearing loss for communication access is a personal hearing device that has been programmed for their specific hearing characteristics and needs. These devices include over-the-counter hearing aids, prescription hearing aids, and cochlear implants. Less often, personal sound amplification products may be used by people with hearing loss.
Deaf individuals may find that personal hearing devices provide very limited access to sound. Because of this minimal benefit and in some cases, cultural preferences, some people who are deaf choose not to wear a personal hearing device. These individuals use technologies that afford visual communication access, such as video calling apps, visual home alerting devices, and television captioning.
Personal Hearing Devices
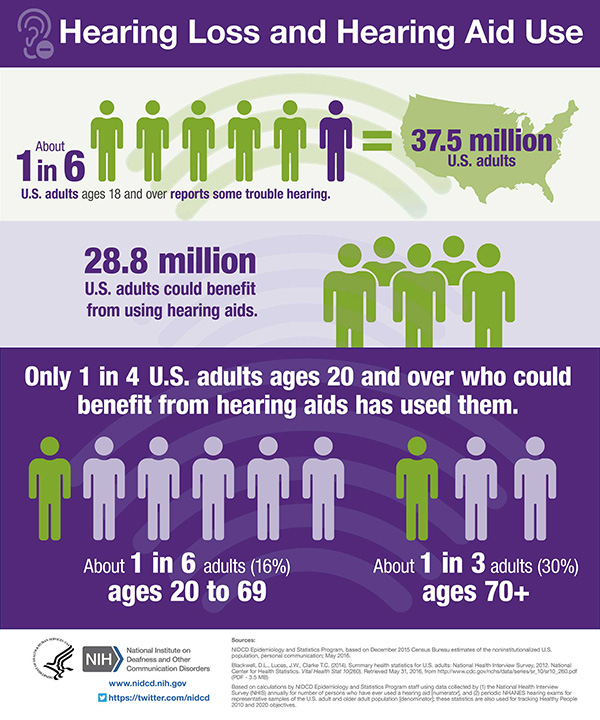
Hearing aids
The Food and Drug Administration (FDA), which regulates hearing aids as medical devices, defines a hearing aid as a “sound-amplifying device designed to aid people who have impaired hearing.” This simple definition obscures the very sophisticated devices that modern hearing aids have become. Hearing Tracker provides a guide to hearing aid manufacturers, brands, and features. The FDA has defined two regulatory categories of hearing aids: Over-the-Counter Hearing Aids and Prescription Hearing Aids. Both are electronic medical devices. They differ in terms of their intended users and conditions for sale.
Over-the-Counter Hearing Aids: The FDA defines over-the-counter (OTC) hearing aids as hearing aids for adults with perceived mild-to-moderate hearing loss that may be purchased directly by consumers without the involvement of a licensed professional. OTC hearing aids are regulated by the FDA with clear labeling as to use, safety and efficacy. This option allows for affordable and easily accessible hearing aids.
Prescription Hearing Aids: The FDA defines prescription hearing aids as hearing aids for people of any age and any degree of hearing loss. A prescription is needed to purchase these devices, and in some states, they must be purchased from a licensed professional.
Cochlear implants
The FDA also regulates cochlear implants, which it defines as “electronic hearing devices.” These devices are very complex, consisting of an external microphone and sound processor that typically sits behind the ear and a surgically implanted electrode array placed in the cochlea of the inner ear. Sound picked up by the microphone is processed and transmitted to the electrode array, where electrical impulses are delivered as a representation of sound.
Personal Sound Amplification Products
Personal sound amplification products (PSAP) are considered consumer electronics products rather than medical devices. They are intended to enhance typical hearing, not address hearing loss. Nevertheless, individuals with hearing loss may choose to purchase and use a PSAP to accommodate their personal hearing needs. PSAPs are not regulated by the FDA. However, the FDA does have non-binding guidance on PSAPs that includes recommendations and their current thinking on this type of device.




